Plankton, so important for our oxygen and to absorb our CO2 production, is in danger.

PCB’s, DDT, PFOS, Dioxins, Methyl mercury, oxybenzone or microplastics… We don’t see most of what is harming marine life – and ours.
Since the 1950s, the chemical revolution, it has decreased by 50%, and it continues to decrease with 1% per year. Reduction of plankton is most likely caused by chemicals that kill the plankton and the coral reef.

Vivid colors of coral become increasingly rare
Even very small amounts of PCB’s, DDT, PFOS, Dioxins, Methyl mercury and UV-filters like oxybenzone are toxic for marine life. Also add plastic to this list, because either these substances are a component in plastics, or they will easily bind to it in the environment.

46,000 pieces of macro plastic per square kilometer, killing more then 1,000,000 seabirds and 100,000 marine mammals, whales and seals every year.
Microplastics adsorb many of the chemicals and concentrate them by the thousands. Plankton eat micro plastics. This is why the plankton are dying at a rate of 1% year.

This is a shrimp. Every shrimp, fish, or living organism in the oceans contain chemicals and 1 in 15 contain micro-plastic.
A sour story of the oceans
CO2 is highly soluble. It dissolves in seawater. Then it forms carbonic acid. And with less plants and plankton in the water to absorb the CO2, the amount of carbonic acid in seas grows. Organisms can’t live in an environment with much acid. So, more plants and plankton die, et cetera.
Before 1950 the ocean was constantly alkalic (the opposite of acidic). But since then, it is acidifying rapidly. The acidification will soon come to a critical point where many life forms cannot survive.
The IPCC worked out several scenarios for the acidification. If we continue our present consumption and production (scenario RCP8.5), the acidification will continue. Only with the strictest scenario on reducing CO2, methane and sulphur dioxide (RCP2.6) we can turn the tide – if we start now.
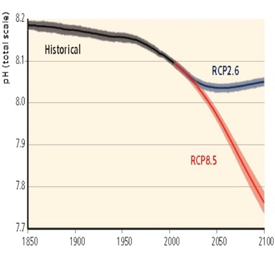
Before the chemical revolution in the 1950s the pH of the oceans was 8.2 to 8.3. Currently, the oceanic pH is about pH 8.06. The acidification will continue to a pH of 7.9-7.95 in about 25 years. From then, most carbonate-based life forms will not be able to survive.
Dominos falling
With many marine life dying, the ecosystem will change rapidly. Like a set of dominos: when one group of organisms dies, another group depending on it, will die, and so on, through the complete food chain. After a couple of years, we will then lose all the whales, seals, birds, fish as well as the food supply for a big part of our world population.

Magnificent marine life, something we can only watch in aquaria in the years to come?
We humans, at the end of the food chain, lose our life support system. The survival of the next generation, of the humanity, comes into question. This is not supposition, or even a hypothesis. When we continue as we do, the dominos will fall in 25 years.
This is about us, not only about whales
Plankton are the lungs of the planet upon which we all depend, but toxic chemicals are killing them.
You and me, we, all the people, in industry, government and households, have 10 years to stop pollution of the ocean. Now we can prevent an immense loss of marine life, and life on earth, including human life. This is not about whales and dolphins, but about you, me and our beloved ones.
So, there’s work to be done. Read about it in our next blog on the warning from the oceans.
Interested?
If you are interested in more literature, follow our links in the text or check out www.goesfoundation.com, where this blog is based on.
Previous blogs in this series:

